From balancing our bodily functions to maintaining clean surfaces, neutralisation reactions play a vital role in daily life. These chemical reactions help counteract the effects of acids and bases, ensuring that everything from personal care to industrial processes runs smoothly.
While you may associate chemistry neutralisation with laboratory experiments, it is a process that occurs constantly in our homes, workplaces, and even within our bodies. In fact, it plays a vital role in biological functions like maintaining the pH level of blood, as well as in many industrial processes.
Understanding neutralisation reactions can give a clearer picture on the essential nature of some household and industrial chemicals. Be it through improvements to agricultural productivity or helping us deal with indigestion, these reactions are an influence on countless aspects of modern life.
In this post:
Key Takeaways
Neutralisation is a chemical reaction that happens between an acid and a base, creating a neutral substance and often also forming water and a salt
Everyday neutralisation reactions occur in personal care, household cleaning, medicine, and even food production
Many common chemicals, such as bleach, fertilisers, and stomach acid treatments, rely on neutralisation to function effectively
Industries, including agriculture, food production, and pharmaceuticals, use neutralisation to enhance efficiency and safety
Understanding neutralisation helps improve safety when using household and industrial chemicals
What is neutralisation in chemistry?
Neutralisation in chemistry is the reaction between a basic and an acidic substance in an aqueous solution. Their respective pH levels (the former is above seven on the pH scale while the latter is below seven) effectively try to cancel each other out.
These types of acid-base reactions almost always produce salt and water. 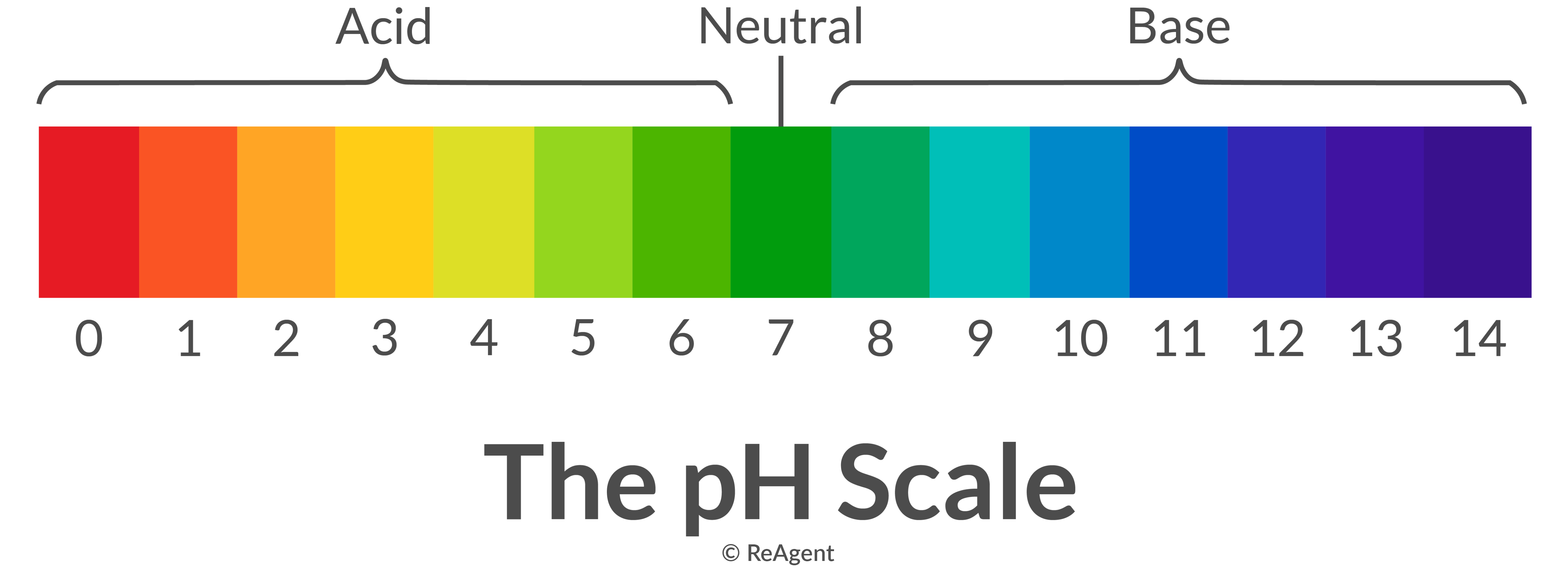
What is a neutralisation reaction?
When an acidic substance is mixed with a basic or alkaline substance in an aqueous solution, a neutralisation reaction occurs. The acidic solution donates protons, while the basic solution accepts protons. Hydronium and hydroxide ions are also removed in the process. The hydroxide ions become water molecules as they combine with the extra protons (hydrogen ions).
Generally, if the chemical reaction is perfectly balanced, the pH of the solution becomes neutral. However, when a strong acid reacts with a weak base, an acidic salt is produced instead. The opposite is also true – if a strong base reacts with a weak acid, a basic salt is produced.
- Example of a strong acid-weak base reaction – the reaction between hydrochloric acid and ammonia produces ammonium chloride, as shown below:
HCl + NH3 → NH4Cl
- Example of a strong base-weak acid reaction – acetic acid and sodium hydroxide react to produce sodium acetate, as shown below:
HC2H3O2 + NaOH → NaC2H3O2 + H2O
Common neutralising chemicals used at home
Many of the products we use for everyday household chores rely on neutralisation reactions. They’re often necessary to either control the potency of a substance or to create other desired substances. Here are some common examples:
1. Bleach
There are two types of laundry bleach products – one contains hydrogen peroxide as the main active ingredient, while the other contains sodium hypochlorite. Typically, household bleach products have a 3-6% sodium hypochlorite concentration by mass, which is usually sufficient to remove stains and kill microbes.
Bleach has a notoriously strong, pungent smell that can be neutralised with hydrogen peroxide, as shown in the equation below. 
NaOCl + H2O2 → O2 + NaCl + H2O
Please note that, for safety’s sake you should never mix highly-concentrated hydrogen peroxide with bleach as it results in a highly exothermic reaction. For a safer option, follow the procedure here on how to gently neutralise the smell of bleach.
2. Methylated, turpentine & white spirits
Methylated, turpentine, and white spirits are all commonly used as paint thinners. Turpentine is a solvent derived from tree resin and consists mainly of monoterpenes α-pinene and β-pinene.
White spirits are petroleum hydrocarbons, while methylated spirits are ethanol mixed with methanol (also known as denatured alcohol). These substances have a strong irritating smell that can be neutralised using acetic acid.
3. Fertilisers
Many organic and inorganic fertilisers are produced using sulphuric acid. For example, ammonium sulphate is a salt produced as a byproduct of the reaction between ammonia and sulphuric acid.
NH3 + H2SO4 → (NH4)2SO4
Neutralisation reactions in everyday life
Neutralisation reactions are part of everyday life. They play a role in everything from biological systems to daily household chores and industrial processes. Here are some examples.
1. Tooth decay
When you consume sugary food or drinks, the bacteria in your mouth multiply very quickly and produce an acid as a metabolic waste product. The acid then reacts with the calcium compounds in your teeth, causing tooth decay. The good news is this can be neutralised by brushing your teeth with toothpaste. Toothpaste contains basic substances, such as calcium carbonate and aluminium hydroxide, which help to neutralise the acid. 
2. Shampoo & conditioner
Shampoos and conditioners contain basic substances such as sodium lauroyl sarcosinate, sodium lauryl sulphate, and sodium laureth sulphate, which act as surfactants. These products are manufactured using neutralisation reactions to make them gentler on the scalp.
3. Stomach acid
Our stomachs produce hydrochloric acid as part of the digestion process. However, if too much acid is produced, hyperacidity may occur. The condition causes digestive discomfort and, in some cases, stomach ulcers. Hyperacidity can be remedied by taking antacids that neutralise excess acid and restore the stomach to its normal pH level (between 1.5 to 3.5). 
Antacid medications may contain one or more of the following ingredients:
- Aluminium hydroxide
- Magnesium carbonate
- Calcium carbonate
- Sodium bicarbonate
Neutralisation reactions in industrial settings
Now that we’ve covered the everyday examples of neutralisation, we’re ready to go into the industrial settings. From agricultural impact to pharmaceutical products, this process is key to helping things work as they should.
Neutralisation in agriculture
Agriculture heavily relies on neutralisation to manage soil health and optimise plant growth. Many crops struggle in overly acidic or alkaline soils, as extreme pH levels can limit nutrient availability and hinder root development.
Farmers use neutralising agents such as lime (calcium carbonate) to balance acidic soils, making essential nutrients like potassium and nitrogen more accessible to plants. Similarly, in cases where soil is too alkaline, substances such as sulphur or ammonium-based compounds can be applied to restore balance.
Neutralisation is also crucial in fertiliser production. Some fertilisers contain acidic or alkaline components that must be adjusted to ensure they do not harm crops. By carefully managing pH levels, farmers can maintain healthier soil conditions, improve water retention, and promote stronger crop yields.
Without neutralisation techniques, agricultural productivity would be significantly impacted, leading to reduced food production and increased reliance on chemical treatments to correct imbalances.
Neutralisation in the food industry
The food industry depends on neutralisation reactions to maintain the quality, safety, and taste of many products. Acidity regulators, such as citric acid and sodium bicarbonate, are widely used to control pH levels in foods and beverages. This is particularly important in preserving perishable items, as excess acidity or alkalinity can affect texture, flavour, and shelf life.
This reaction, often used in baking and brewing, also relies on neutralisation to happen. For example, in bread-making, acidic ingredients like vinegar or lemon juice interact with baking soda to produce carbon dioxide, which helps dough rise.
Meanwhile in dairy production, neutralisation is essential in balancing the acidity of milk and cheese products to ensure proper texture and taste. Without careful pH control, many food products would spoil more quickly or fail to develop the desired consistency.
Neutralisation in the pharmaceutical industry
The pharmaceutical industry uses neutralisation in the formulation of many medicines. Antacids, for example, are designed to neutralise excess stomach acid, providing relief from conditions such as heartburn and acid reflux.
These medications typically contain basic compounds like magnesium hydroxide or calcium carbonate, which react with stomach acid to form water and a neutral salt, reducing discomfort.
Neutralisation is also vital in pharmaceutical manufacturing, where precise pH control ensures the stability and effectiveness of active ingredients. Many medications must be formulated within a specific pH range to be absorbed properly by the body.
Additionally, pharmaceutical companies use neutralisation reactions to control the acidity of intravenous solutions, ensuring they are safe for direct entry into the bloodstream. Without careful pH regulation, many drugs would not work as they should, or could cause unwanted side effects.
The importance of neutralisation reactions in daily life
The importance of neutralisation reactions in daily life can’t be underestimated.
Understanding neutralisation is essential for both practical and scientific reasons, from improving agricultural efficiency to maintaining health and hygiene.
Neutralisation improves the effectiveness of many everyday products and also ensures that potentially harmful substances are rendered harmless. Greater awareness of these processes can lead to more informed decisions when handling chemicals at home and in professional settings.
Conclusion
Neutralisation reactions play a significant role in everyday life, ensuring substances remain safe, effective, and balanced. By recognising their importance in personal care, cleaning, medicine, and industry, we can better appreciate the science behind many daily activities. From preventing tooth decay to the mass production of fertilisers, neutralisation has a crucial role to play.












