A standard solution is any chemical solution that’s been prepared from a primary standard and where you know the concentrations.
It typically has a relatively high molar mass that can be accurately measured for comparison. It must also have a high level of purity, stability, and solubility in water.
A standard solution is measured to a fixed volume as per your requirements. To prepare a standard solution, you need an accurate weighing scale that’s correctly calibrated at the milligram level. You’ll also require a graduated cylinder that can measure fractions of millilitres.
Standard solutions play an important role in laboratory analysis and manufacturing processes. Purity tests and chemical reaction tests, such as acid-base neutralisation (titration experiment) require a standard solution.
Standard solutions can also be used to quality control mass-produced products and test for pollution based on a certain threshold.
In this post:
The Characteristics of Standard Solutions
A standard solution can consist of any solute-solvent combination if the precise concentrations are known. Water is commonly used as a solvent, while a water-soluble compound usually acts as the solute. 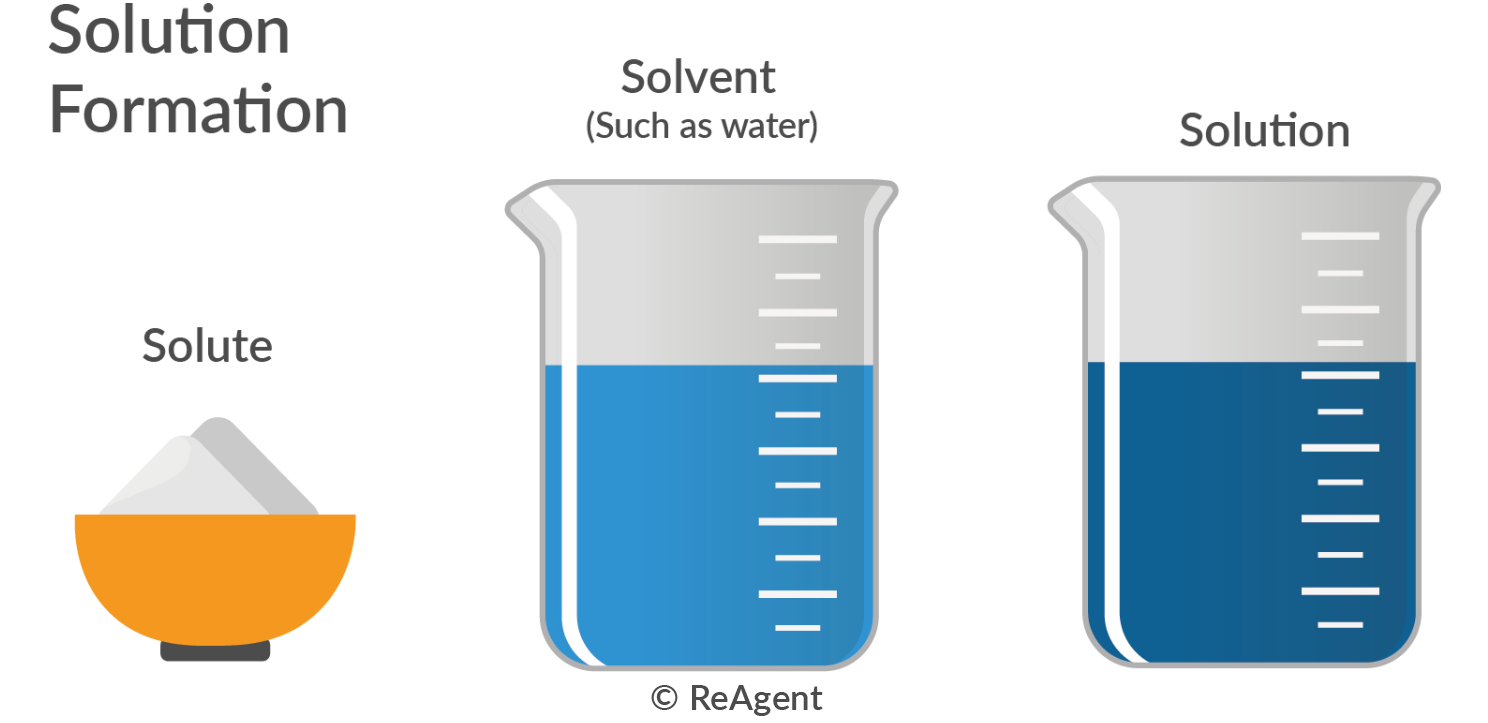
Standard solutions can either be bought from a chemical supplier or they can be manufactured to suit your needs. However, not all substances can be prepared into standard solutions. A standard solution must have the following characteristics:
- High level of purity – No other extraneous substances should be present in the solution or, at the very least, any impurities must be negligible. This is crucial when you require accurate and precise measurements.
- Non-reactive with air or water – If the solute reacts with air or water it will no longer be the same substance and you won’t achieve the desired results.
- Solubility – The solute must be reasonably soluble in water to achieve the necessary molarity. It must not simply act as a suspension that settles down as the solid precipitates.
- High molar mass – This is important because you need to weigh the solute and compare it with the molarity of the substance you’re testing. You may need to test for the specific gravity of the analyte based on the known specific gravity of the solute in the standard solution.
- Non-hygroscopic property – This means the solute does not absorb moisture from the air. Its weight can therefore be maintained at a precise range even if the humidity fluctuates.
What are Primary Standard Solutions?
A primary standard solution is a type of reagent that has a high level of purity. It has a very precise concentration that reflects the molar concentration of the solute. Its pure state means you can measure the mass to volume ratio with a high degree of accuracy.
Aside from its high purity level, which is typically more than 99%, the solute substance is stable and non-reactive to air and water. Not all substances are suited to being part of a standard solution due to their relative chemical instability.
For example, sodium hydroxide (NaOH) can absorb carbon dioxide from the air, which affects its concentration as a solute.

A primary standard solution can be used to calibrate secondary standard solutions. Some examples of primary standard solutions include:
- Sodium chloride – table salt is used as a primary standard solution for reactions involving silver nitrate.
- Powdered zinc – powdered zinc is often used to standardise ethylenediaminetetraacetic acid or EDTA, which acts as a stabiliser in many pharmaceutical products. The zinc powder is dissolved in either hydrochloric or sulphuric acid as a primary standard solution.
- Potassium hydrogen phthalate – this can be used to calibrate perchloric acid and acetic acid as standard solutions.
What are Secondary Standard Solutions?
Secondary standard solutions aren’t as pure or as chemically stable as primary standard solutions. Unlike primary standards, secondary standard solutions may react slightly with water and air. They’re also hygroscopic, which means they may need to be calibrated before being weighed.
Secondary standards solutions are used for specific analytical applications including:
- Acid-base titrations
- Reduction-oxidation titrations
- Precipitation titrations
- Complexometric titrations

Secondary standard solutions are used in conducting acid-base titration experiments
How do you Prepare a Standard Solution in Chemistry?
To prepare a standard solution, you first need to weigh a standard solute based on the desired molar concentration. You’ll then need to dissolve the solute in a specific volume of solvent. You can measure and adjust the concentration using parameters such as pH level, the specific gravity, spectrometry and assay tests.
How is a Standard Solution Used in Analytical Chemistry?
Standard solutions have various applications in analytical chemistry. As we’ve already discussed, primary standard solutions can be used to calibrate secondary standard solutions. Both primary and secondary solutions are also integral to the qualitative analysis of solutions with unknown concentrations.
Standard solutions are primarily used in titration analysis to determine the concentration of analytes based on the threshold of neutralisation. As reagents, these solutions are crucial in synthesising various products, including medicines. They can also help to maintain quality control in products.













