Condensation is when a substance changes from a gaseous or vapour state into a liquid state. You can think of it as the reverse of evaporation. Condensation isn’t exclusively limited to water – it can also occur in many other substances in vapour forms. However, in this article we’ll be focusing on water condensation.
Condensation is a natural process that occurs because of a change in temperature. It can occur on a small scale, such as water vapour in the air condensing into water droplets on the surface of a cold window. But when condensation occurs on a large scale, it leads to precipitation or rain. On this scale, it is part of the global water cycle, which is the largest source of condensation.
In this post:
What Does Condensation Mean?
Condensation is the opposite of evaporation. It’s a physical change that involves a substance’s transition from gaseous form into liquid form. Usually, this is caused by a high to low temperature change. At the same time, condensation is affected by many other factors, such as pressure, presence of condensation nuclei or surface, and airflow.
Just like evaporation, water condensation has a direct role in weather and the climate. It’s an intermediate stage between evaporation and precipitation. An everyday example of condensation is the dew drops that form on plant life before sunrise, especially when fog settles on the ground. Dew drops accumulate on surfaces that have lower temperatures than the fog itself – such as blades of grass.
But a surface doesn’t necessarily have to be lower in temperature than the vapour in order for condensation to occur. For example, condensation commonly occurs on condensation nuclei in the air. Condensation nuclei are fine particles that are at least one micrometre in diameter. They are suspended in the air at the same levels as clouds. They can be ash particles from volcanoes or they can be salt particles from the oceans. Interestingly, many condensation nuclei are also particles from meteors that are burned up upon entry into the atmosphere.

What Happens During Condensation?
If you’ve ever boiled water in a kettle, you’ve probably noticed the droplets of water that form on the bottom surface of the lid. These droplets are water condensations from the steam. Similarly, you’ve probably observed dew drops on your lawn grass every morning, or droplets on your window pane during winter. Again, these are all examples of water condensations.
In order for condensation to occur, some conditions must be met. These conditions don’t necessarily have to occur simultaneously. In many cases, one condition is sufficient.
- Change in temperature: Some temperature difference is needed in order for condensation to occur. For example, the surface of a cold soda can easily accumulates water droplets once you take it out of the refrigerator. Similarly, in winter, the temperature difference between your warm home and the wintry air outside can cause condensation to occur on your windows.
- Presence of condensation nuclei: As we mentioned earlier, condensation nuclei are very small particles suspended in the air at cloud level. They include smoke particles from fires, ashes from volcanic eruptions, and dust particles from burned up meteors. These particles are hygroscopic, which means that they attract water molecules.
- Change in pressure: Pressure has an inverse proportional relationship with evaporation. As pressure decreases, the rate of evaporation increases. This is because the molecules of a fluid can move more freely. Conversely, pressure has a direct proportional relationship with condensation. As pressure increases, condensation rate also increases.
- A surface area: A surface can be the surface of a leaf, a rock, or a metal. Wider surfaces have greater capacity to accumulate condensation.
- Humidity: Humidity is the water vapour content in air. Higher humidity means a greater chance of condensation, especially if there is a temperature drop.
What is an Example of Condensation?
The best and largest example of condensation is the clouds. Cloud formations are simply mist, or very fine particles of water, suspended in the air. When the droplets converge into larger droplets, they become too heavy to remain as a fine mist. Therefore, the droplets fall or precipitate as rain. Heavier clouds (like cumulonimbus clouds) look more opaque, dark, and dense because water droplets are converging into larger droplets.

Is Condensation Preventable?
Condensation is a necessary natural process, and it’s generally desirable. However, there are some instances where condensation is not desirable because it’s potentially destructive or even life threatening.
For example, you would not want sudden condensation on your windscreen when you’re driving. The windscreen can potentially become foggy and semi-opaque if there is high humidity inside and low temperatures outside. You can prevent window fogging by cleaning your windshield and removing damp items inside the car. You can also use silica dehumidifiers inside your car.
Condensation is also undesirable inside buildings, especially on walls and ceilings. Aside from causing paint cracks, indoor condensation can lead to structural damage. Wooden structures, mouldings, and plaster on walls and ceilings are particularly vulnerable to destructive condensation.
The potential dangers and destructive effects of condensation indoors can be prevented by controlling the various factors of internal climate. You should control the humidity, temperature, and airflow to prevent undesirable condensation.
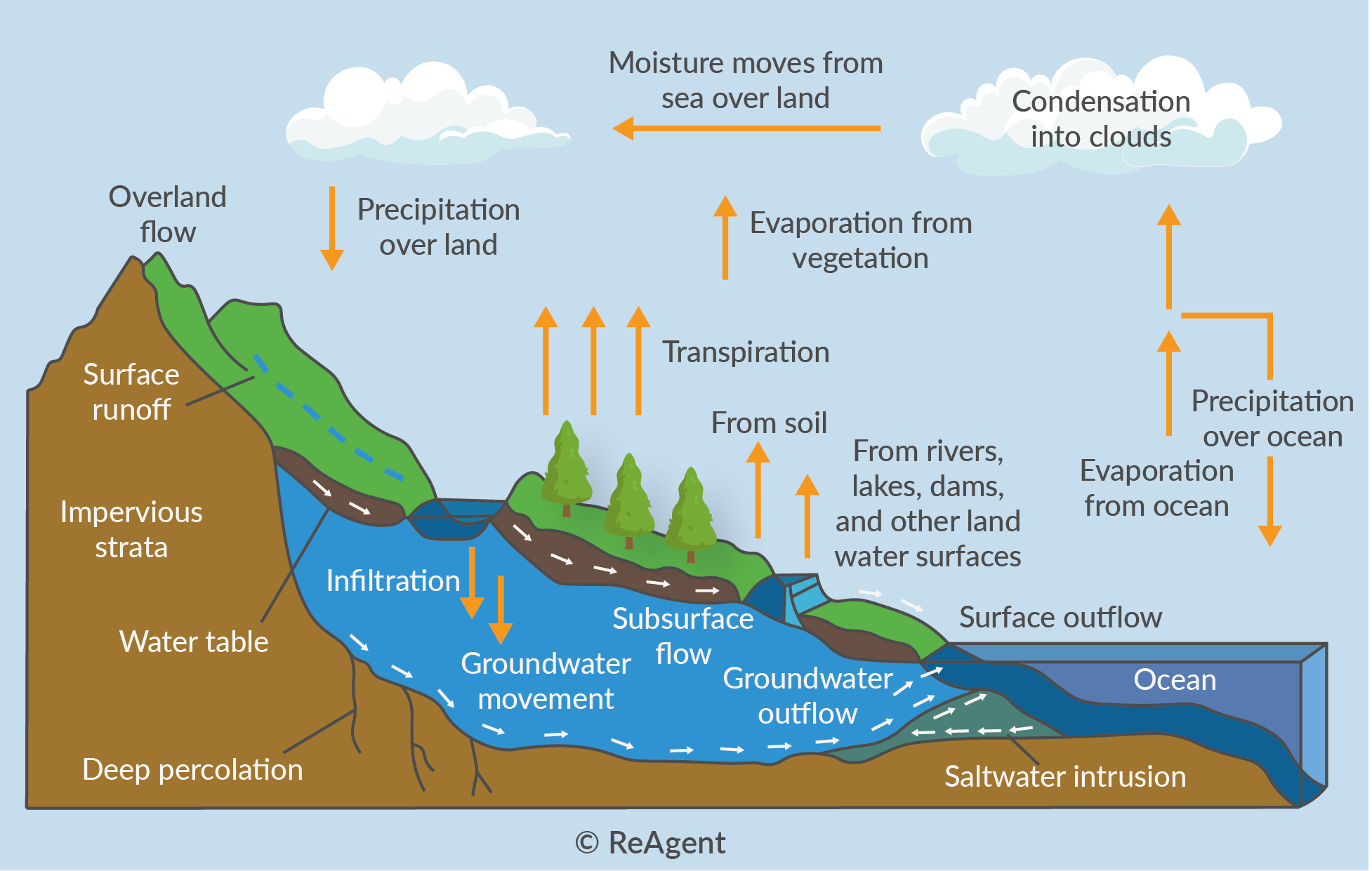
What is Condensation in the Water Cycle?
Cloud formation and dew formation are condensation stages in the water cycle. Of course, the most important stage is cloud formation because without clouds, precipitation in the form of rain or snow would not be possible.
As we touched on earlier, condensation is the intermediate stage between evaporation and precipitation. Clouds distribute rain in large areas away from the main source of evaporation, which is the ocean. The centres of large continents that have fewer clouds usually become deserts and are too arid for regular rainfalls.
What’s the Difference Between Condensation and Evaporation?
Although condensation and evaporation are both physical processes of phase changes, they are opposite processes. Condensation requires lower temperatures, higher pressure, and higher humidity. On the other hand, evaporation requires higher temperatures and lower pressure as ideal conditions.
With evaporation, liquid states begin to transition into vapour states as temperatures increase – think about a puddle evaporating in the sun. Meanwhile, condensation involves a substance in a vapour state transitioning into a liquid state.












