Isopropanol, which is also known as isopropyl alcohol (IPA) and propan-2-ol, has a range of applications across many industries. It comes in various grades, each one having different uses. It is also one of the most common compounds of the alcohol family, and one of the most widely-used solvents in the world.
In this post:
What is Isopropanol?
Firstly, let’s look at what isopropanol actually is. Most commonly known as rubbing alcohol, isopropanol a colourless, flammable liquid with a strong, distinctive smell. With the chemical formula C3H8O, isopropanol is a secondary alcohol. This means that its alcohol carbon atom is attached to two other carbon atoms.
Isopropanol was first produced by hydrating propene in 1920 at Standard Oil, a large oil refinery. It was discovered whilst studying by-products of petroleum. Nowadays, it can be produced in three different ways: indirect hydration of propylene, direct hydration of propylene, and catalytic hydrogenation of acetone.
Uses of Isopropanol
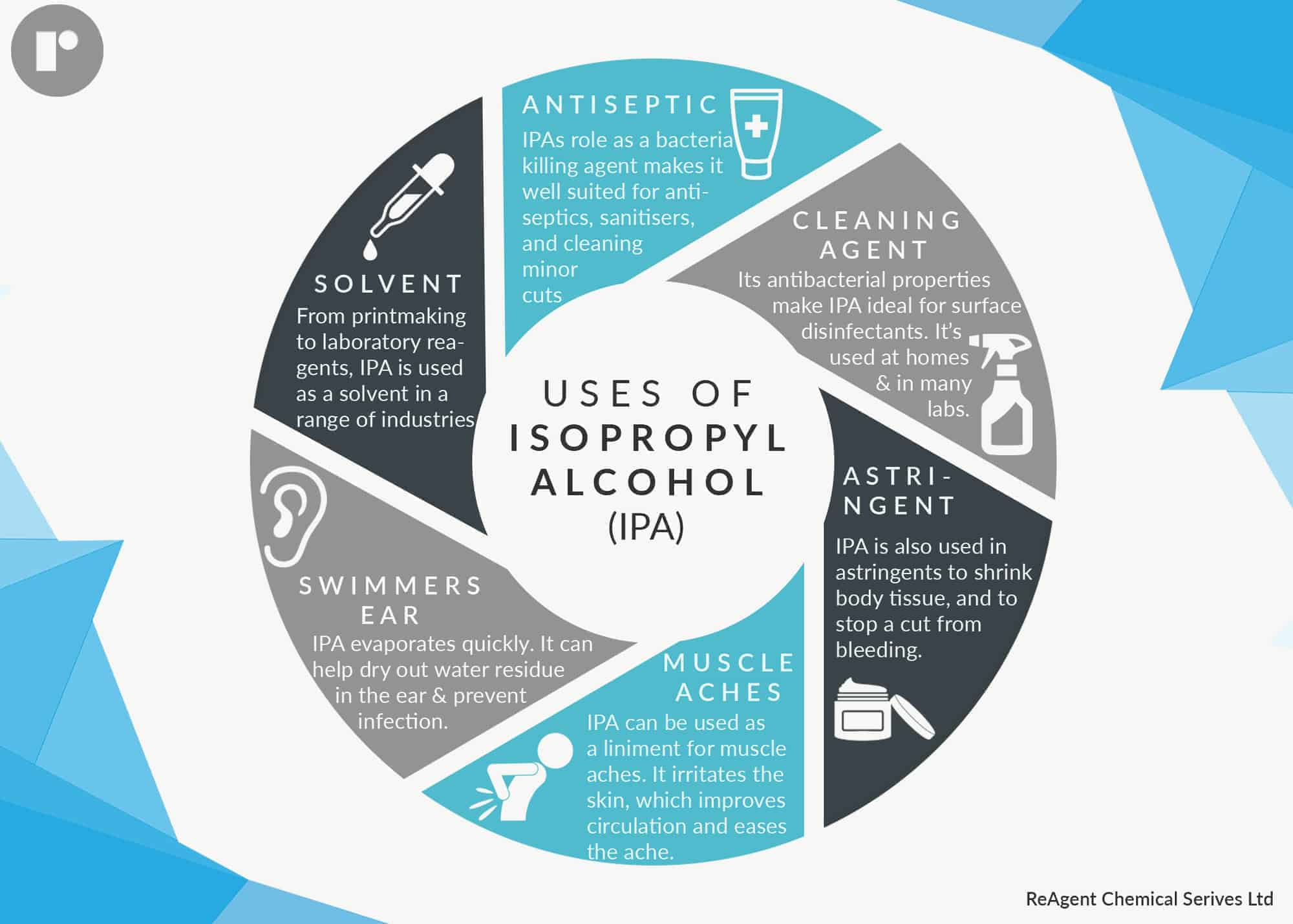
Isopropanol has many uses, some of which we’ll look at individually: as a solvent, an antiseptic, a cleaning agent, an astringent, and for muscle aches.
Isopropanol as a Solvent
Isopropanol dissolves a wide range of compounds. It evaporates quickly, leaves nearly zero residue compared with ethanol, is relatively non-toxic, and can be mixed with most solvents including water, ethanol, and chloroform. Therefore it is used widely as a solvent.
When isopropanol is mixed with water it creates rubbing alcohol, also known as surgical spirit. In this form it has a wide range of home-based uses from disinfecting cuts to de-icing vehicle windscreens.
Isopropanol is also used in the home for disinfecting surfaces and even removing permanent marker.
As an industrial solvent, isopropanol is used in a variety of different processes, including:
- Stripping paint
- Equipment cleaning in printmaking
- Dilution and extraction in laboratory chemicals
Using Isopropanol as an Antiseptic
Isopropanol has a high concentration of alcohol of between 70% and 99%. While that makes it unsafe to drink, 70% alcohol is an effective antiseptic. This is because at that concentration, isopropanol can penetrate the cell wall and kill a microorganism. It then also protects against surface bacteria.
It is used by doctors to clean your skin before you have an injection, as an ingredient in hand sanitisers, and on small cuts and scrapes.
Isopropanol – the Cleaning Agent
Isopropanol is an ingredient in many hospital surface cleaning and disinfectant products. It’s even on the Environmental Protection Agency’s list of Antimicrobial Products Effective Against Mycobacterium Tuberculosis, Human HIV-1 and Hepatitis B Virus.
That means it’s also very effective in labs, for cleaning electrical components, and in home cleaning products. As it leaves no residue, it’s often an ingredient in glass cleaners, and is great for cleaning mirrors as it doesn’t leave streaks.

Astringent
Astringents cause body tissue to tighten. Isopropanol is an ingredient in many skin toners because it acts as an astringent by causing pores to contract, leaving skin looking smoother.
It can also be used to stop bleeding from minor cuts and scrapes (as well as disinfecting the wound at the same time), because it makes blood capillaries contract.
Isopropanol Eases Muscle Ache
Isopropanol dries out skin to an extent that it is classified as a skin irritant, which is why you shouldn’t use too much of it if you’re using it as an antiseptic. It’s this same effect that makes ispropanol good for muscle ache.
It’s often an ingredient in liniments simply because it irritates the skin, and therefore increases blood flow in that part of your body. In turn, this improved blood circulation eases muscle ache and inflammation.
At ReAgent, we manufacture isopropanol in a range of sizes and grades, including analytical, laboratory, general and 15% v/v. Contact our expert team today to discuss your isopropanol needs.