Methyl red is an azo dye, or a synthetic dye, which is one of the common chemical indicators used in the laboratory to determine pH transitions within a specific range. It’s most effective between pH 4.4 and pH 6.2.
If the pH level is below 4.4, its colour is red, and if it’s above 6.2, the colour turns yellow. If the pH is somewhere between these two limits, the colour is orange. This change in colour also corresponds to the extent by which the protons or hydronium ions (H+) dissociate from the molecules of the dye.
Methylene red is useful when it comes to testing the pH changes in acidic solutions. It’s also useful for calibrating solutions, especially during titration experiments. It isn’t, however, effective for testing pH changes in alkaline solutions because it remains yellow in colour when mixed with them.
In this post:
What is the Chemical Formula of Methylene Red?
Azo dyes have a generalised chemical formula and structure that can be written as R−N=N−R. The hydrocarbon chains or rings are typically aryl groups, which are derived from aromatic compounds.
The IUPAC standard name of methyl red is 2-(N,N-dimethyl-4-aminophenyl) azobenzenecarboxylic acid, but it’s also known as C.I. Acid Red 2. It has a molar mass of 269.304 g·mol−1. When it isn’t dissolved in water, the dye is a dark red crystalline powder at room temperature. Its simple chemical formula can be written as C15H15N3O2.

Together with murexide, methyl red is being investigated for its potential for the sonochemical destruction of chlorinated hydrocarbon pollutants. Sonochemistry is a relatively new field of research that focuses on the chemical reactions that occur with the application of powerful ultrasonic waves, specifically ones with frequencies between 20 kHz and 10 MHz.
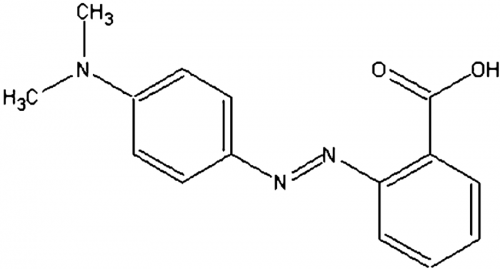
Structurally, the chemical composition and molecular relationships of the elements in methyl red can be illustrated as:
Methyl red can be prepared through the diazotisation of anthranilic acid, which is followed by a chemical reaction with dimethylaniline. The series of chemical processes is illustrated below:
What is Methyl Red Used For?
Azo dyes have important commercial value as dyes for textiles, leather products, and some food products.
Aside from common laboratory titration experiments, methyl red is also used in microbiology, where it can identify bacteria that produce stable acids through the mixed acid fermentation of glucose. This test identifies enteric bacteria based on the pattern of glucose metabolism. Enteric bacteria are clinically important because they’re pathogenic, with Salmonella and E. coli being some of the most common examples that are pathogenic to humans.
Enterics or bacteria that belong to the Enterobacteriaceae family have glucose metabolic pathways that initially produce pyruvic acid. The mixed acid pathway is used by some enterics to convert pyruvic acid into other types of acids, such as lactic acid, acetic acid, and formic acid.
Methyl red can be used to identify these bacteria based on their acidic metabolic byproducts. Therefore, they’re called methyl-red positive bacteria, and this term includes things like E. coli and P. vulgaris. On the other hand, methyl-red negative bacteria such as S. marcescens and E. aerogenes use the butylene glycol pathway to convert pyruvic acid into neutral metabolic byproducts.
The process of testing for enteric bacteria starts with:
- An isolate that’s inoculated into a tube using a sterile transfer loop
- The second step is to incubate the tube at 35°C for two to five days
- Next, the incubated medium is transferred into another tube
- Finally, five drops of methyl red are added to the medium
- The mixture is then gently rolled between the palms to ensure the indicator is properly dispersed
Once the methyl red is properly dispersed into the medium, it’s expected to turn red if the pH is below 4.4. The presence of pyruvic acid that’s metabolised into other acids with pH readings of at least 4.2 will produce the positive test results for the presence of pathogenic enterics like Escherichia coli. On the other hand, if the pH level of the medium is only 6.0, then the test result is negative, and the indicator will be yellow in colour.

Why is Methyl Red Used in Titration?
Titration is the process of slowly adding a solution of known concentration and known volume (the titrant) to a solution of unknown concentration and known volume (the analyte). This is done until a threshold concentration is achieved, which is determined by the colour change of an indicator, like methyl red. Usually, the change in colour indicates neutralisation, but it can also mean the desired threshold has been reached, depending on the experiment.
Four types of titration methods are developed as analytical tools for determining the unknown concentration of a solution. The analyte is of known chemical species that’s also known to react with the titrant. These are the four categories of titration:
- Acid-base titrations
- Redox titrations
- Precipitation titrations
- Complexometric titrations
While the substances, specific goals, and exact procedures may vary, all titrations can be classified as one of the aforementioned categories. For example, methyl red can be used in the spectroscopy analysis of inorganic compounds. Methyl red is good for the titration of a strong acid (analyte) with a strong base. The lower pH range makes it ideal for acid titration.